
SL Paper 3
of the mass of a rock weighing is uranium(IV) oxide, . of the uranium atoms in the rock are uranium-238, .
Show that the mass of the isotope in the rock is .
The half-life of 238U is years. Calculate the mass of 238U that remains after has decayed for years.
Outline a health risk produced by exposure to radioactive decay.
Deduce the nuclear equation for the decay of uranium-238 to thorium-234.
Thorium-234 has a higher binding energy per nucleon than uranium-238. Outline what is meant by the binding energy of a nucleus.
The mild analgesic aspirin can be prepared in the laboratory from salicylic acid.
(CH3CO)2O + HOC6H4COOH → CH3CO2C6H4COOH + CH3COOH
Salicylic acid Aspirin
After the reaction is complete, the product is isolated, recrystallized, tested for purity and the experimental yield is measured. A student’s results in a single trial are as follows.
Literature melting point data: aspirin = 138–140 °C
Determine the percentage experimental yield of the product after recrystallization. The molar masses are as follows: M(salicylic acid) = 138.13 g mol−1, M(aspirin) = 180.17 g mol−1. (You do not need to process the uncertainties in the calculation.)
Suggest why isolation of the crude product involved the addition of ice-cold water.
Justify the conclusion that recrystallization increased the purity of the product, by reference to two differences between the melting point data of the crude and recrystallized products.
State why aspirin is described as a mild analgesic with reference to its site of action.
An investigation was carried out to determine the effect of chain length of the alcohol on the equilibrium constant, , for the reversible reaction:
The reactants, products and the catalyst form a homogeneous mixture.
Fixed volumes of each alcohol, the ethanoic acid and the sulfuric acid catalyst were placed in sealed conical flasks.
At equilibrium, the flasks were placed in an ice bath, and samples of each flask titrated with to determine the ethanoic acid concentration present in the equilibrium mixture.
The following processed results were obtained.
© International Baccalaureate Organization 2020
Identify the independent and dependent variables in this experiment.
The ice bath is used at equilibrium to slow down the forward and reverse reactions. Explain why adding a large amount of water to the reaction mixture would also slow down both reactions.
Suggest why the titration must be conducted quickly even though a low temperature is maintained.
An additional experiment was conducted in which only the sulfuric acid catalyst was titrated with . Outline why this experiment was necessary.
Calculate the percentage uncertainty and percentage error in the experimentally determined value of for methanol.
Comment on the magnitudes of random and systematic errors in this experiment using the answers in (e).
Suggest a risk of using sulfuric acid as the catalyst.
In a class experiment, students were asked to determine the value of x in the formula of a hydrated salt, BaCl2・xH2O. They followed these instructions:
- Measure the mass of an empty crucible and lid.
- Add approximately 2 g sample of hydrated barium chloride to the crucible and record the mass.
- Heat the crucible using a Bunsen burner for five minutes, holding the lid at an angle so gas can escape.
- After cooling, reweigh the crucible, lid and contents.
- Repeat steps 3 and 4.
Their results in three trials were as follows:
State and explain the further work students need to carry out in trial 2 before they can process the results alongside trial 1.
In trial 3, the students noticed that after heating, the crucible had turned black on the outside. Suggest what may have caused this, and how this might affect the calculated value for x in the hydrated salt.
List two assumptions made in this experiment.
Students were asked to investigate how a change in concentration of hydrochloric acid, HCl, affects the initial rate of its reaction with marble chips, CaCO3.
They decided to measure how long the reaction took to complete when similar chips were added to 50.0 cm3 of 1.00 mol dm−3 acid and 50.0 cm3 of 2.00 mol dm−3 acid.
Two methods were proposed:
(1) using small chips, keeping the acid in excess, and recording the time taken for the solid to disappear
(2) using large chips, keeping the marble in excess, and recording the time taken for bubbles to stop forming.
A group recorded the following results with 1.00 mol dm−3 hydrochloric acid:
Annotate the balanced equation below with state symbols.
CaCO3(__) + 2HCl(__) → CaCl2(__) + CO2(__) + H2O(__)
Neither method actually gives the initial rate. Outline a method that would allow the initial rate to be determined.
Deduce, giving a reason, which of the two methods would be least affected by the chips not having exactly the same mass when used with the different concentrations of acid.
State a factor, that has a significant effect on reaction rate, which could vary between marble chips of exactly the same mass.
Justify why it is inappropriate to record the uncertainty of the mean as ±0.01 s.
If doubling the concentration doubles the reaction rate, suggest the mean time you would expect for the reaction with 2.00 mol dm−3 hydrochloric acid.
Another student, working alone, always dropped the marble chips into the acid and then picked up the stopwatch to start it. State, giving a reason, whether this introduced a random or systematic error.
Body fluids have different pH values.
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
Outline how ranitidine reduces stomach acidity.
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
Ethanol was electrolysed at different voltages. The products at the anode, ethanoic acid, ethanal and carbon dioxide, were collected and analysed.
The percentages of products obtained using three different catalysts mounted on a carbon anode, platinum (Pt/C), platinum and ruthenium alloy (PtRu/C) and platinum and tin alloy (PtSn/C) are shown.
Chemical yields of ethanoic acid, ethanal and carbon dioxide as a function of voltage for
oxidation of 0.100 mol dm−3 ethanol at Pt/C, PtRu/C and PtSn/C anodes at 80°C.
[Source: Product Distributions and Efficiencies for Ethanol Oxidation in a Proton Exchange Membrane Electrolysis Cell, Rakan M. Altarawneh and Peter G. Pickup, Journal of the Electrochemical Society, 2017, volume 164, issue 7, http://jes.ecsdl.org/. Distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/)]
Describe the effect of increasing the voltage on the chemical yield of:
Ethanal using Pt/C:
Carbon dioxide using PtRu/C:
Determine the change in the average oxidation state of carbon.
From ethanol to ethanal:
From ethanol to carbon dioxide:
List the three products at the anode from the least to the most oxidized.
Deduce, giving your reason, which catalyst is most effective at fully oxidizing ethanol.
Palmitic acid has a molar mass of 256.5 g mol−1.
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.
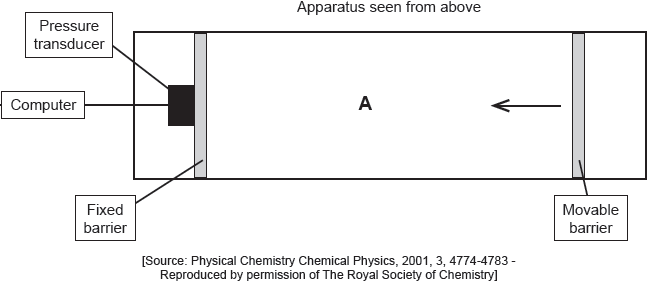
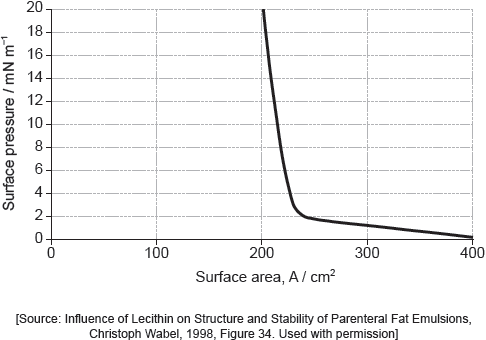
When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.
The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
State and explain how the graph would differ if 1 moldm−3 sulfuric acid had been used instead of 1 moldm−3 hydrochloric acid.
Alloys containing at least 60 % copper reduce the presence of bacteria on their surface.The percentage of copper in brass, an alloy of copper and zinc, can be determined by UV-vis spectrometry.
A sample of brass is dissolved in concentrated nitric acid and then made up to 250.0 cm3 with water before analysis.
Cu (s) + 4HNO3 (aq) → Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O (l)
3Zn (s) + 8HNO3 (aq) → 3Zn(NO3)2 (aq) + 2NO (g) + 4H2O (l)
The concentration of copper(II) ions in the resulting solution is then determined from a calibration curve, which is plotted by measuring the light absorbance of standard solutions.
Titration is another method for analysing the solution obtained from adding brass to nitric acid.
Outline why the initial reaction should be carried out under a fume hood.
Deduce the equation for the relationship between absorbance and concentration.
Outline how a solution of 0.0100 mol dm−3 is obtained from a standard 1.000 mol dm−3 copper(II) sulfate solution, including two essential pieces of glassware you would need.
The original piece of brass weighed 0.200 g. The absorbance was 0.32.
Calculate, showing your working, the percentage of copper by mass in the brass.
Deduce the appropriate number of significant figures for your answer in (d)(i).
Comment on the suitability of using brass of this composition for door handles in hospitals.
If you did not obtain an answer to (d)(i), use 70 % but this is not the correct answer.
Suggest another property of brass that makes it suitable for door handles.
Copper(II) ions are reduced to copper(I) iodide by the addition of potassium iodide solution, releasing iodine that can be titrated with sodium thiosulfate solution, Na2S2O3 (aq). Copper(I) iodide is a white solid.
4I− (aq) + 2Cu2+ (aq) → 2CuI (s) + I2 (aq)
I2 (aq) + 2S2O32− (aq) → 2I− (aq) + S4O62− (aq)
Deduce the overall equation for the two reactions by combining the two equations.
Suggest why the end point of the titration is difficult to determine, even with the addition of starch to turn the remaining free iodine black.
Consider the following lipid and carbohydrate.
In order to determine the number of carbon-carbon double bonds in a molecule of linoleic acid, 1.24 g of the lipid were dissolved in 10.0 cm3 of non-polar solvent.
The solution was titrated with a 0.300 mol dm–3 solution of iodine, I2.
Determine the empirical formula of linoleic acid.
The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per gram than fructose.
State the type of reaction occurring during the titration.
Calculate the volume of iodine solution used to reach the end-point.
Outline the importance of linoleic acid for human health.
Physical properties of elements vary according to atomic number. Sections 6 to 9 of the data booklet list some of these properties.
Deduce, giving a reason, the group of elements in the periodic table most likely to undergo sublimation.
Describe the density trend across periods 4 and 5 of the periodic table.
Suggest, with a reason, whether the lanthanoids or actinoids of the f-block would have the higher density.
Compare the ease of oxidation of s-block and d-block metals to their melting points and densities. Use section 25 of the data booklet.
Sketch how the first ionization energies of elements vary with their atomic radius.
Sodium chloride, NaCl, can be spread on icy roads to lower the freezing point of water.
The diagram shows the effects of temperature and percentage by mass of NaCl on the composition of a mixture of NaCl and H2O.
Estimate the lowest freezing point of water that can be reached by adding sodium chloride.
Estimate the percentage by mass of NaCl dissolved in a saturated sodium chloride solution at +10 ºC.
Calculate the percentage of water by mass in the NaCl•2H2O crystals. Use the data from section 6 of the data booklet and give your answer to two decimal places.
Suggest a concern about spreading sodium chloride on roads.
Describe the characteristics of the nematic liquid crystal phase and the effect that an electric field has on it.
Shape of molecules:
Distribution:
Effect of electric field:
Consider the following antacids:
Show that antacid X is more effective, per tablet, than antacid Y.
Disposable plastic lighters contain butane gas. In order to determine the molar mass of butane, the gas can be collected over water as illustrated below:
List the data the student would need to collect in this experiment.
Explain why this experiment might give a low result for the molar mass of butane.
Suggest one improvement to the investigation.
Aspirin is one of the most widely used drugs in the world.
Aspirin was synthesized from 2.65 g of salicylic acid (2-hydroxybenzoic acid) (Mr = 138.13) and 2.51 g of ethanoic anhydride (Mr = 102.10).
Calculate the amounts, in mol, of each reactant.
Calculate, in g, the theoretical yield of aspirin.
State two techniques which could be used to confirm the identity of aspirin.
State how aspirin can be converted to water-soluble aspirin.
Compare, giving a reason, the bioavailability of soluble aspirin with aspirin.
Gasoline (petrol), biodiesel and ethanol are fuels.
[U.S. Department of Energy. https://afdc.energy.gov/]
Calculate the energy released, in , from the complete combustion of of ethanol.
State a class of organic compounds found in gasoline.
Outline the advantages and disadvantages of using biodiesel instead of gasoline as fuel for a car. Exclude any discussion of cost.
A mixture of gasoline and ethanol is often used as a fuel. Suggest an advantage of such a mixture over the use of pure gasoline. Exclude any discussion of cost.
Contrast the molecular structures of biodiesel and the vegetable oil from which it is formed.
When combusted, all three fuels can release carbon dioxide, a greenhouse gas, as well as particulates. Contrast how carbon dioxide and particulates interact with sunlight.
Methane is another greenhouse gas. Contrast the reasons why methane and carbon dioxide are considered significant greenhouse gases.
Suggest a wavenumber absorbed by methane gas.
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
Suggest how the end point of the titration might be estimated from the graph.
This question is about a mug made of a lead alloy.
The rate of lead dissolving in common beverages with various pH values was analysed.
Identify the experiment with the highest rate of lead dissolving.
Suggest why the relationship between time and lead concentration for Cola at 16 °C is not linear.
Lead(II) chloride, PbCl2, has very low solubility in water.
PbCl2 (s) Pb2+ (aq) + 2Cl− (aq)
Explain why the presence of chloride ions in beverages affects lead concentrations.
A mean daily lead intake of greater than 5.0 × 10−6 g per kg of body weight results in increased lead levels in the body.
Calculate the volume, in dm3, of tap water from experiment 8 which would exceed this daily lead intake for an 80.0 kg man.